
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Pathophysiology
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,232 View
- 185 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
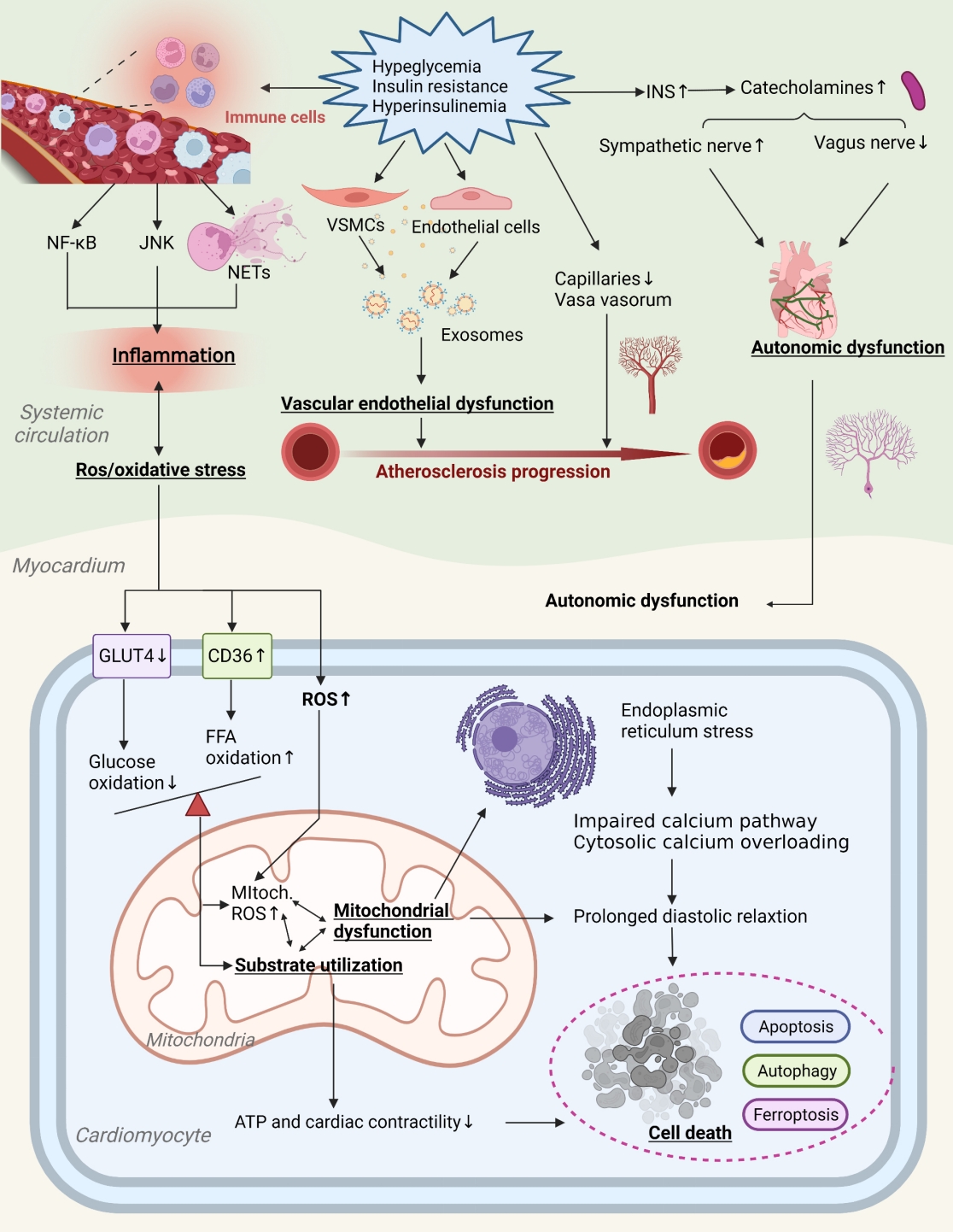
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Metabolic Risk/Epidemiology
- A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women
- Qiong Chen, Weiwei Wu, Hailan Yang, Ping Zhang, Yongliang Feng, Keke Wang, Ying Wang, Suping Wang, Yawei Zhang
- Diabetes Metab J. 2020;44(6):887-896. Published online September 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0138
- 6,602 View
- 135 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Identification of modifiable dietary factors, which are involved in the development of gestational diabetes mellitus (GDM), could inform strategies to prevent GDM.
Methods
We examined the dietary patterns in a Chinese population and evaluated their relationship with GDM risk using a case-control study including 1,464 cases and 8,092 control subjects. Propensity score matching was used to reduce the imbalance of covariates between cases and controls. Dietary patterns were identified using factor analysis while their associations with GDM risk were evaluated using logistic regression models.
Results
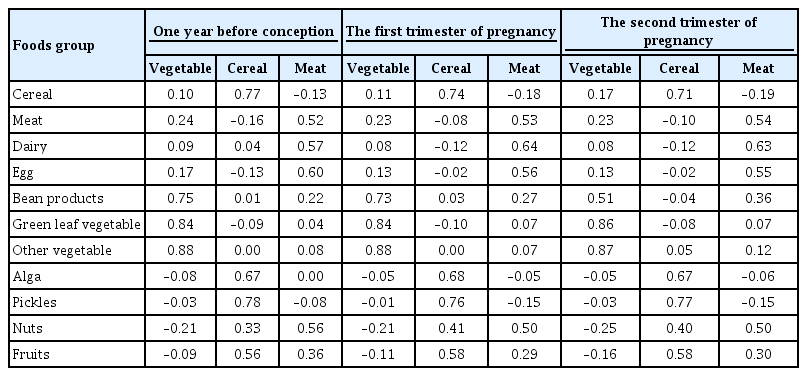
A “vegetable” dietary pattern was characterized as the consumption of green leafy vegetables (Chinese little greens and bean seedling), other vegetables (cabbages, carrots, tomatoes, eggplants, potatoes, mushrooms, peppers, bamboo shoots, agarics, and garlic), and bean products (soybean milk, tofu, kidney beans, and cowpea). For every quartile increase in the vegetables factor score during 1 year prior to conception, the first trimester, and the second trimester of pregnancy, the GDM risk lowered by 6% (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.89 to 0.99), 7% (OR, 0.94; 95% CI, 0.88 to 0.99), and 9% (OR, 0.91; 95% CI, 0.86 to 0.96).
Conclusion
In conclusion, our study suggests that the vegetable dietary pattern is associated with lower GDM risk; however, the interpretation of the result should with caution due to the limitations in our study, and additional studies are necessary to explore the underlying mechanism of this relationship. -
Citations
Citations to this article as recorded by- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health
Victoria Lambert, Sonia Edith Muñoz, Carla Gil, María Dolores Román
Nutrition Journal.2023;[Epub] CrossRef - Fruit, vegetable, and fruit juice consumption and risk of gestational diabetes mellitus: a systematic review and meta-analysis
Yan-Ping Liao, Qing-Xiang Zheng, Xiu-Min Jiang, Xiao-Qian Chen, Xiao-Xia Gao, Yu-Qing Pan
Nutrition Journal.2023;[Epub] CrossRef - The effects of plant-based dietary patterns on the risk of developing gestational diabetes mellitus: A systematic review and meta-analysis
Yu Zhu, QingXiang Zheng, Ling Huang, XiuMin Jiang, XiaoXia Gao, JiaNing Li, RuLin Liu, Kent Lai
PLOS ONE.2023; 18(10): e0291732. CrossRef - Molecular pathways and nutrigenomic review of insulin resistance development in gestational diabetes mellitus
Patricia Guevara-Ramírez, Elius Paz-Cruz, Santiago Cadena-Ullauri, Viviana A. Ruiz-Pozo, Rafael Tamayo-Trujillo, Maria L. Felix, Daniel Simancas-Racines, Ana Karina Zambrano
Frontiers in Nutrition.2023;[Epub] CrossRef - Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies
Swetha Sampathkumar, Durga Parkhi, Yonas Ghebremichael-Weldeselassie, Nithya Sukumar, Ponnusamy Saravanan
Nutrition & Diabetes.2023;[Epub] CrossRef - Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies
Elaine Luiza Santos Soares de Mendonça, Marilene Brandão Tenório Fragoso, Jerusa Maria de Oliveira, Jadriane Almeida Xavier, Marília Oliveira Fonseca Goulart, Alane Cabral Menezes de Oliveira
Antioxidants.2022; 11(1): 129. CrossRef - Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in db/db mice
Jie Gao, Xue Gu, Manqian Zhang, Xingwang Zu, Fukui Shen, Xiaotao Hou, Erwei Hao, Gang Bai
Journal of Functional Foods.2022; 91: 105009. CrossRef - Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance
Jianping Wang, Zuoliang Xie, Peipei Chen, Yuhuan Wang, Baoqing Li, Fen Dai
Open Life Sciences.2022; 17(1): 202. CrossRef - Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women
Weijia Wu, Nu Tang, Jingjing Zeng, Jin Jing, Li Cai
Nutrients.2022; 14(8): 1623. CrossRef - Dietary Acid Load Is Positively Associated With Risk of Gestational Diabetes Mellitus in a Prospective Cohort of Chinese Pregnant Women
Rui Zhao, Leilei Zhou, Gang Lei, Shanshan Wang, Yan Li, Xuefeng Yang, Guoping Xiong, Liping Hao
Frontiers in Nutrition.2022;[Epub] CrossRef
- Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health
- Pathophysiology
- Relationships between Islet-Specific Autoantibody Titers and the Clinical Characteristics of Patients with Diabetes Mellitus
- Yiqian Zhang, Tong Yin, Xinlei Wang, Rongping Zhang, Jie Yuan, Yi Sun, Jing Zong, Shiwei Cui, Yunjuan Gu
- Diabetes Metab J. 2021;45(3):404-416. Published online July 21, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0239
- 6,391 View
- 161 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Dysimmunity plays a key role in diabetes, especially type 1 diabetes mellitus. Islet-specific autoantibodies (ISAs) have been used as diagnostic markers for different phenotypic classifications of diabetes. This study was aimed to explore the relationships between ISA titers and the clinical characteristics of diabetic patients.
Methods A total of 509 diabetic patients admitted to Department of Endocrinology and Metabolism at the Affiliated Hospital of Nantong University were recruited. Anthropometric parameters, serum biochemical index, glycosylated hemoglobin, urinary microalbumin/creatinine ratio, ISAs, fat mass, and islet β-cell function were measured. Multiple linear regression analysis was performed to identify relationships between ISA titers and clinical characteristics.
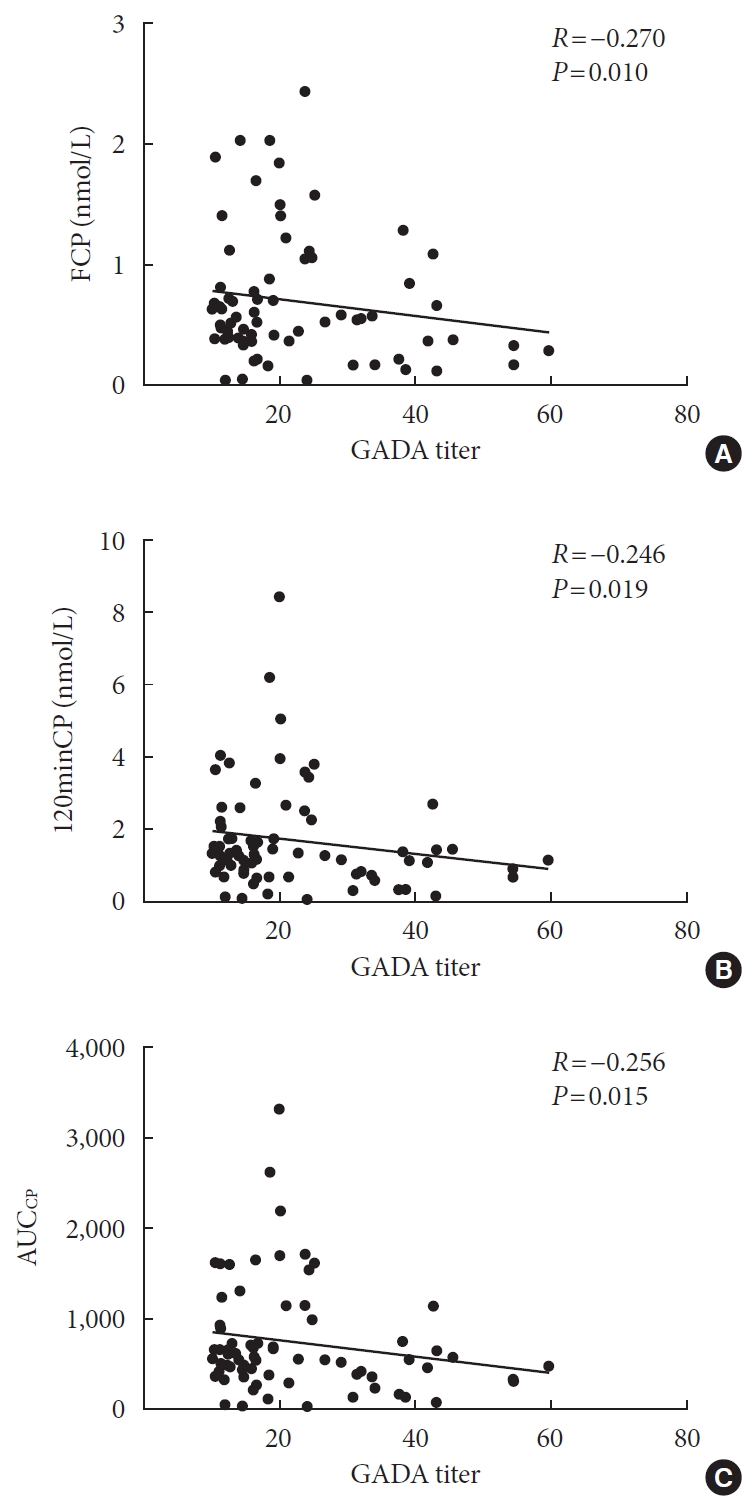
Results Compared with autoantibody negative group, blood pressure, weight, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), visceral fat mass, fasting C-peptide (FCP), 120 minutes C-peptide (120minCP) and area under C-peptide curve (AUCCP) of patients in either autoantibody positive or glutamate decarboxylase antibody (GADA) positive group were lower. Body mass index (BMI), waist circumference, triglycerides (TGs), body fat mass of patients in either autoantibody positive group were lower than autoantibody negative group. GADA titer negatively correlated with TC, LDL-C, FCP, 120minCP, and AUCCP. The islet cell antibody and insulin autoantibody titers both negatively correlated with body weight, BMI, TC, TG, and LDL-C. After adjusting confounders, multiple linear regression analysis showed that LDL-C and FCP negatively correlated with GADA titer.
Conclusion Diabetic patients with a high ISA titer, especially GADA titer, have worse islet β-cell function, but less abdominal obesity and fewer features of the metabolic syndrome.
-
Citations
Citations to this article as recorded by- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults
Tomislav Bulum, Marijana Vučić Lovrenčić, Jadranka Knežević Ćuća, Martina Tomić, Sandra Vučković-Rebrina, Lea Duvnjak
Biomedicines.2023; 11(9): 2561. CrossRef - RETRACTED ARTICLE: MiRNA-27a mediates insulin resistance in 3T3-L1 cells through the PPARγ
Yangming Zhuang, Ming Li
Molecular and Cellular Biochemistry.2022; 477(4): 1107. CrossRef - Correlation between Insulin Resistance and Microalbuminuria Creatinine Ratio in Postmenopausal Women
Han Na, Rong Wang, Hai-Long Zheng, Xiao-Pan Chen, Lin-Yang Zheng, Faustino R. Perez-Lopez
International Journal of Endocrinology.2022; 2022: 1. CrossRef - The longitudinal loss of islet autoantibody responses from diagnosis of type 1 diabetes occurs progressively over follow-up and is determined by low autoantibody titres, early-onset, and genetic variants
C L Williams, R Fareed, G L M Mortimer, R J Aitken, I V Wilson, G George, K M Gillespie, A J K Williams, Chitrabhanu Ballav, Atanu Dutta, Michelle Russell-Taylor, Rachel Besser, James Bursell, Shanthi Chandran, Sejal Patel, Anne Smith, Manohara Kenchaiah,
Clinical and Experimental Immunology.2022; 210(2): 151. CrossRef
- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults

 KDA
KDA
 First
First Prev
Prev





